Multiplet Splitting – 3s – Orbitals
MULTIPLET SPLITTING of “3s” Orbitals (split final states)
Multiplet Splitting occurs in core level XPS whenever there is one (or more) unpaired electron(s) in the valence levels. Multiplet splitting occurs due to the exchange interaction between the unpaired valence electrons and the unpaired electron left in the core level (after photoionization). This interaction produces “split final states”.
In other words:
Multiplet splitting arises when an atom contains unpaired valence electrons. When a core electron vacancy is created by photoionization, there can be coupling between the remaining unpaired electron in the core with the unpaired electrons in the outer shell. This can create a number of final states, which will be seen in the photoelectron spectrum as a multi-peak envelop. The Figure below shows the multiplet splitting structure that exists for the
Multiplet Splitting (split final states) occurs for compounds having unpaired valence electrons interacting with:
- 3s electrons in materials such as: MnO, MnF2, CrF2, …
- 2p electrons in materials such as: CuO, CuSO4…
- 3d electrons in rare earth compounds such as: CeO2…
- Gaseous compounds exhibit multiplet splitting of the 1s orbital, such as: NO, NNO…
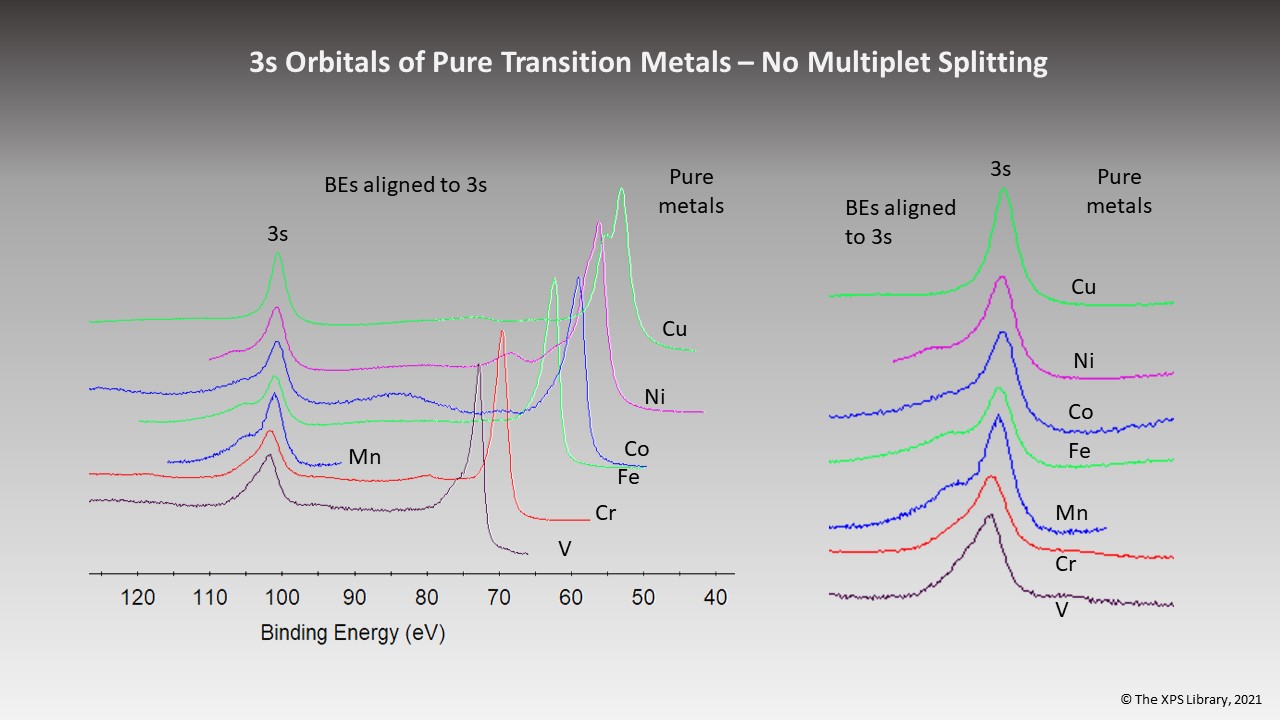
Pure Transition Metals – No “3s” Multiplet Splitting for Pure Metals
Electron Diagram for Multiplet Splitting of “3s” Orbitals in Chemical Compounds


Important Notes about “3s” XPS from Paul Bagus:
1. The TM 3s XPS is dominated by atomic effects. There are quantitative changes for metals and compounds but the basic physics is atomic physics.
2. The 3s XPS cannot be explained with a one-configuration model of multiplets. The Mn splitting from a simple exchange splitting model is 3X larger than observed; For other methods see the Viinikka paper.
3. The concept of FAC (Frustrated Auger Configuration) provides a powerful way to understand the many body effects that complicate the 3s XPS
4. Compelling proof of the character of the 3s XPS in magnetically ordered Fe was obtained from spin-polarized XPS; see paper with Mallow. It would be nice if the experiments could be extended to the region where the satellites are observed.
5. It is not possible to understand the 3s (or the 3p) XPS unless a many electron, many configuration model is used.
6. I view the spectra as arising from XPS forbidden configurations stealing intensity from the XPS allowed configurations. Pure theorists do not like this description because it isn’t perfect but it conveys the physics clearly and correctly.
7. The 3p XPS is even more complicated than the 3s XPS.
Spectra from Transition Metal Compounds – Having Multiplet Splitting in “3s” Orbital

| Iron (3s) Final State Multiplet Splitting FeF2 solid Iron (Fe) atom electron configuration 1s2 2s2 2p6 3s2 3p6 3d6 4s2 2 un-paired electrons in 3d orbital |
||
| Manganese (3s) Final State Multiplet Splitting MnO, MnF2, Mn (gas) Manganese (Mn) atom electron configuration 1s2 2s2 2p6 3s2 3p6 3d5 4s21 un-paired electrons in 3d orbital |
||
| Manganese (3s) Final State Multiplet Splitting MnF2, MnO, and MnO2 Manganese (Mn) atom electron configuration 1s2 2s2 2p6 3s2 3p6 3d5 4s21 un-paired electrons in 3d orbital |
||
| Copper (3s) Final State Multiplet Splitting CuO solid Copper (Cu) atom electron configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s11 un-paired electrons in 4s orbital |
|
|
| Comparison of “3s” Multiplet Splittings
for FeF2, MnF2, and CrF2 |
 |
|
|
|
|
|
|
|
|
|
| Oxygen (1s) Final State Multiplet Splitting
Mixture of O2 gas and H2O gas Oxygen atom electron configuration 2 unpaired electrons O2 is Paramagnetic |
|
|
| Nitrogen (1s) Final State Multiplet Splitting
Nitrous Oxide, NO, gas Nitrogen atom electron configuration 1 unpaired electron NO is Paramagnetic |
 |
|
